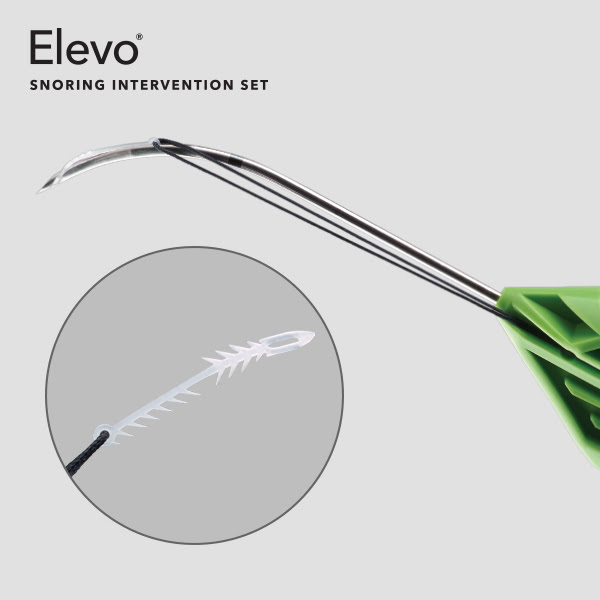
Effective procedure
After the lifting and stiffening action created in the soft palate, patients experienced a 30.3% reduction in sVAS at 180 days post-procedure.1
Office-based approach
The Elevoplasty® procedure can be performed under local anesthesia in an office setting without the need for surgery.
Ease of use
The set comes pre-assembled in a sterile tray, which only requires the physician to open the package; the physician can be ready to perform the procedure from beginning to end without having to prepare or assemble the device in advance.
Elevo and Elevoplasty are registered trademarks of Zelegent, Inc.1. Elevo kit snoring intervention device. Premarket notification 510(k) K181107.
This email was sent by Ear, Nose & Throat Journal on behalf of Cook Medical, 750 N. Daniels Way, Bloomington, IN 47402-0489 USA. OHNS-D50070-EN
|



Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου