| Pulmonary complications in liver disease
Chronic liver disease and impaired hepatic function are important risk factors for increased incidence of, and mortality from, acute pulmonary complications including manifest acute respiratory distress syndrome in approximately 8% of cirrhotics admitted to an intensive care unit (ICU) [1, 2]. While micro-aspiration associated with encephalopathy and hydropic decompensation are well-known triggers for pulmonary complications [3], the more specific consequences of end-stage liver disease on the pulmonary microcirculation, such as hepatopulmonary syndrome (HPS) and portopulmonary hypertension (PoPH), result from either production of or failure to clear a broad range of inflammatory, vasoactive or proliferative/angiogenic mediators [4]. The impact of an altered bile acid pattern induces a shift in the gut microbiome [5] that sheds new light on the molecular basis of the long acknowledged ‘gut–liver–lung axis’ as the “motor of multiple organ failure”.
Two discrete entities affecting the pulmonary vascular bed—hepatopulmonary syndrome and portopulmonary hypertension
In addition to these acute organ–organ interactions, prolonged stimulation by liver-derived vasoactive and proliferative mediators promotes remodeling processes in the pulmonary vascular bed in chronic liver disease through diffuse or localized telangiectasia (in HPS) or hyperplastic lesions in terminal pulmonary arterioles (in PoPH) [6].
Respiratory function needs to be assessed in all candidates listed or considered for liver transplantation (LT), as primary disorders affecting the pulmonary circulation are associated with functional status, quality of life, and survival on the waiting list and outcome after LT [7, 8]. HPS and PoPH, a form of secondary pulmonary hypertension (PAH), might also prompt unplanned admission of the patient with end-stage liver disease with dyspnea or right heart dysfunction and require immediate and appropriate care on an ICU to prevent further deterioration of an already limited liver function.
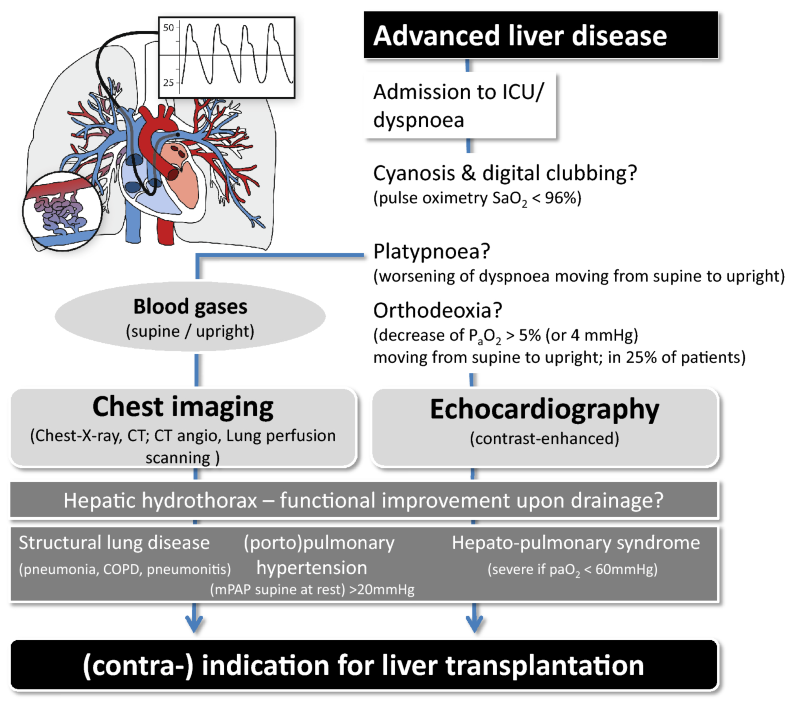
Hepatopulmonary syndrome is characterized by hypoxemia with an abnormal alveolar–arterial O2-gradient and evidence of intrapulmonary shunting in the presence of liver disease [6, 9]. Abnormal oxygenation is defined by elevated alveolar–arterial oxygen gradient (> 15 mmHg or > 20 mmHg in patients > 64 years, respectively) while breathing room air in a sitting position, and severity of HPS is classified according to the degree of hypoxemia [6]. The underlying intrapulmonary vascular dilatations can best be diagnosed by contrast-enhanced echocardiography, and characteristic findings on CT imaging may also be seen. In severe cases, clinical characteristics of hypoxemia such as cyanosis and digital clubbing might be present. The severity of HPS might be aggravated by upright posture (platypnea–orthodeoxia) and is not related to the severity of liver disease. In general, the pulmonary circulation is diffusely affected by telangiectatic lesions, but anatomically defined shunts might less commonly exist that are potentially amenable to interventional therapy (Fig. 1) [6].
Fig. 1
Alterations of the pulmonary vascular bed in chronic liver disease: chronic liver disease promotes remodeling processes in the pulmonary vascular bed characterized by diffuse or localized telangiectasia (in HPS) or hyperplastic lesions in terminal pulmonary arterioles (PoPH). The diagnostic workup includes blood gases in the supine and upright posture and contrast-enhanced echocardiography. Evidence of secondary pulmonary hypertension or right-to-left shunting triggers further imaging. HPS and PoPH are associated with functional status, quality of life, and survival on the waiting list and outcome after LT with strong impact on listing for LT
Hepatopulmonary syndrome is present in 10–30% of patients with cirrhosis being evaluated for LT, but has also been reported in non-cirrhotic portal hypertension and acute liver injury in the critically ill, e.g., in patients after shock events presenting signs of “hypoxic hepatitis” [9]. As such, acute HPS as a consequence of acute and chronic heart failure, in particular failure of the right ventricle with hepatic congestion, can complicate the ICU course through induction of pulmonary shunting (i.e., HPS). This bi-directional cross talk of lung and liver can be viewed as a prominent example of organ–organ interactions that pave the way to multiple organ failure and death.
Supportive therapies with supplemental oxygen and liver transplantation remain the only therapies with proven benefit in cases of HPS associated with end-stage liver disease, and Model of End-Stage Liver Disease (MELD) exception can be granted to facilitate liver transplantation in various programs to reverse two potentially fatal organ dysfunctions [7, 10, 11]. Patients with HPS are at increased risk for prolonged mechanical ventilation with a longer ICU length of stay compared to other liver transplant recipients [12]. Most literature reports addressing management of these critical events reflect case reports or small case series, where inhaled vasodilators, most notably nitric oxide and extracorporeal membrane oxygenation (ECMO), have been applied as rescue strategies [13, 14]. Albeit inhaled NO might selectively dilate well-aerated alveoli, the use of vasodilators in HPS in general remains controversial and should be restricted to short-acting NO in exceptional cases and as compassionate use.
Impaired clearance of vasoactive mediators by the failing liver plays a significant role in the pathogenesis of HPS, and the resulting right-to-left shunting, mismatch between ventilation and perfusion and impaired arterial oxygenation are generally reversible with recovery of hepatic function [7, 8, 10]. As a consequence, persistent severe hypoxemia and features of HPS despite improved liver function might reflect and should prompt search for concomitant anatomic shunting. However, in severe cases of HPS, remodeling of the pulmonary vasculature and reversal of shunting due to diffuse telangiectasia may take weeks or months. Pre-transplant risk assessment and classification of severity of HPS is mandatory, as patients with a paO2 < 60 mmHg not reversible to 100% oxygen may develop prolonged respiratory failure in the post-transplant period [10, 11].
In contrast, PoPH is a form of secondary pulmonary arterial hypertension occurring in only 2–8% of cirrhotic patients [15] and, as in HPS, it can also be seen in patients with non-cirrhotic portal hypertension. Presenting symptoms include dyspnea and fatigue and signs of increased workload of the right heart, such as elevated jugular venous pulse and tricuspid regurgitation. Other causes for PAH have to be ruled out and patients with PoPH require meticulous optimization of PAH-specific therapies prior to indication for LT. Treatment of PoPH includes inhaled or intravenous prostaglandins and agents such as sildenafil or bosentan, which are improved options to manage pulmonary hypertension and allow facilitating successful LT in this high-risk population. It is essential that the diagnosis of either arterial or venous pulmonary hypertension is identified and the latter may be successfully treated by addressing the volume status. Right heart catheterization is an essential component of assessment following echocardiography. Patients with PoPH have poorer survival when undergoing LT, but transplantation should be considered only in patients with mild PoPH or those responding to medical therapy with pulmonary vasodilators and MPAP < 35 mmHg [10]. In summary, pulmonary complications are important in well-defined subgroups of end-stage liver disease. These patients require meticulous assessment prior to listing and reflect high-risk patients in the peritransplant setting due to the development of right heart or weaning failure, respectively. Vice versa, deterioration of liver function and hypoxic hepatitis as a result of (right) heart failure can aggravate intrapulmonary shunting due to acutely developing HPS, an underestimated phenomenon in the general ICU population.
NotesAcknowledgements
The cartoon in the figure was provided by Dr. M. Leitner from the Center for Sepsis Care & Control, University Hospital Jena, Germany.
Compliance with ethical standardsConflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
|
| When will less monitoring and diagnostic testing benefit the patient more?
The choosing wisely campaign has highlighted for each medical profession the five practices that both physicians and patients should question (http://www.choosingwisely.org/). Achieving informed test selection was named as one of the five challenges our profession should address in the years to come. However, the means of achieving this aim remains unclear. Some call for real-time disclosure of the costs and consequences of excessive testing. Others believe in better education on the topic. Both approaches are challenging, and neither is likely to suffice alone. There is also a need to change the medical system and societal expectations from a good doctor.
We perform many tests simply because we can and because we hesitate to change longstanding routines. We also overtest because we are concerned that we might miss an important finding that will ultimately affect patient survival [1]. However, excessive testing carries a heavy price. Over half of the intensive care unit (ICU) patients...
|
Prognostic relevance of serum lactate kinetics: a powerful predictor but not Chuck Norris in Intensive Care MedicineReferences
|
Traumatic occipitoatlantal dissociation: high index of suspicion should be kept in mindReference
|
| High-flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: author's reply
Dear Editor,
Thanks to Dr. Luo and colleagues for their letter and comments [1]. In the protocol for our systematic review, we decided a priori that all analysis would be conducted using a random effects model, as this analytic plan better accounts for between-study differences [2]. In situations where statistical heterogeneity is minimal, the output from random effects models closely matches that of fixed effects models. However, in response to this letter, we did perform fixed effects analysis for this outcome and the pooled point estimate and 95% confidence intervals do not change.
We focused our TSA analysis on the outcomes most likely to be considered for future RCTs. As escalation of therapy is a composite, including outcomes of variable clinical and patient importance, we intentionally highlighted ‘need for IMV’ and ‘mortality’. For the TSA analysis, we had initially used the relative risk reductions from our pooled analysis to inform the sample size calculation. After peer review of the manuscript, we were asked to choose a threshold consistent with what we believed would be a clinically important difference. As such, we selected a 15% relative risk reduction for this dichotomous outcome.
NotesReferences
|
Confirmation of brain death on VA-ECMO should mandate simultaneous distal arterial and post-oxygenator blood gas samplingReferences
|
| A patient with dermo-chondro-corneal dystrophy (François syndrome) and acute dyspnea |
| Emergency craniotomy in semi-lateral position for posterior fossa hemorrhage evacuation under venoarterial extracorporeal membrane oxygenation |
| Mechanical thrombectomy for a cerebral fat embolism
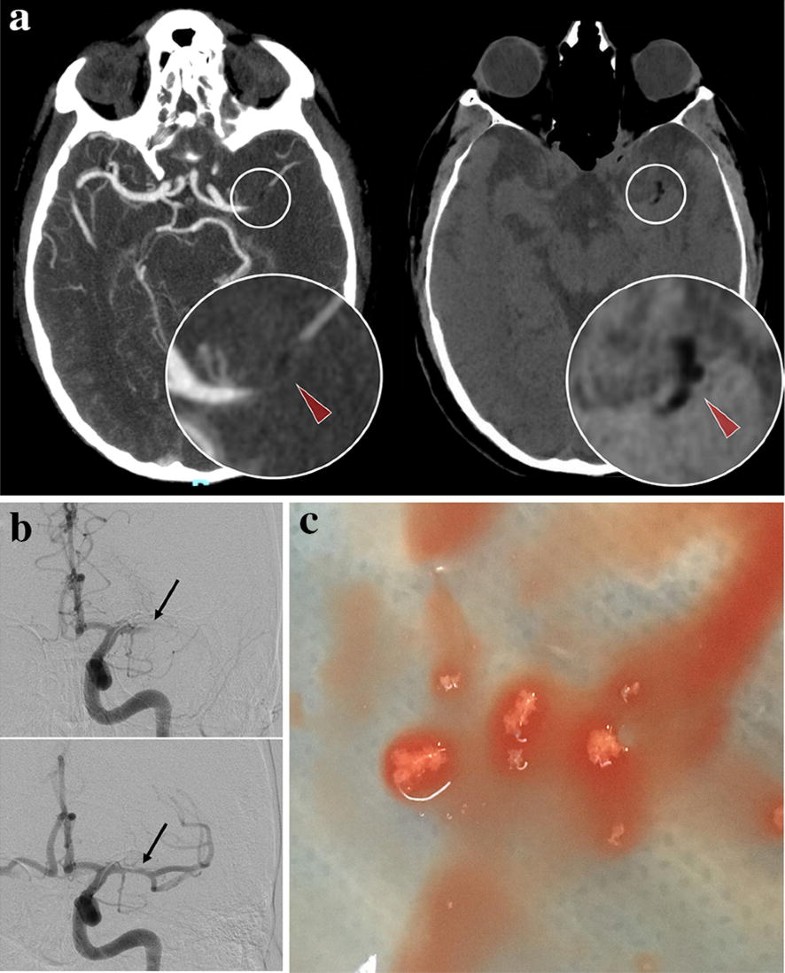
A 56-year-old patient underwent a right knee replacement under spinal anesthesia. At the end of the procedure, he suddenly developed right hemiplegia and aphasia. Emergent brain computed tomography (CT) revealed a left middle cerebral artery occlusion by material of negative density (− 33 Hounsfield Unit), highly suggestive of fat embolus (Fig. 1a). Indeed, this image differs from the typical hyperdense artery sign, which shows thrombus. Mechanical thrombectomy was successfully achieved by thrombo-aspiration (Fig. 1b) and retrieved spumous fat fragments (Fig. 1c). In this context of acute setting during orthopedic surgery, an intra-cardiac right-left shunt was searched during diagnostic work-up. Transesophageal echocardiography revealed a patent foramen ovale with an atrial septal aneurysm, supporting a paradoxical embolism of fat material released during surgery. The patient recovered well.
Fig. 1
a Injected cerebral computed tomography demonstrating left middle cerebral artery occlusion (left panel) due to a negative density embolus (− 33 Hounsfield Units, red arrowheads). b Pre- and post-aspiration angiographic runs showing arterial occlusion (top, black arrow) and complete revascularization of the left middle cerebral artery (bottom, black arrow). c Macroscopic aspect of the fat embolus after thrombectomy by direct intracranial aspiration
Brain CT remains the standard for acute neurological disorders because of its almost universal availability, and speed of acquisition, especially in the context of stroke, where every minute counts. In some cases, brain CT may also provide a pathognomonic clue of embolus constitution and help tailor revascularization strategy, i.e., the use of direct aspiration instead of a stentriever for a deemed friable fat embolus in this case.
|
| A rare cause of laryngeal stenosis |
Medicine by Alexandros G. Sfakianakis,Anapafseos 5 Agios Nikolaos 72100 Crete Greece,00302841026182,00306932607174,alsfakia@gmail.com,
Ετικέτες
Κυριακή 4 Αυγούστου 2019
Intensive Care Medicine August 2019, Volume 45, Issue 8, pp 1152–1153
Αναρτήθηκε από
Medicine by Alexandros G. Sfakianakis,Anapafseos 5 Agios Nikolaos 72100 Crete Greece,00302841026182,00306932607174,alsfakia@gmail.com,
στις
3:40 π.μ.

Ετικέτες
00302841026182,
00306932607174,
alsfakia@gmail.com,
Anapafseos 5 Agios Nikolaos 72100 Crete Greece,
Medicine by Alexandros G. Sfakianakis
Εγγραφή σε:
Σχόλια ανάρτησης (Atom)

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου